Quantum theory of atomic structure pdf
Data: 2.09.2017 / Rating: 4.6 / Views: 645Gallery of Video:
Gallery of Images:
Quantum theory of atomic structure pdf
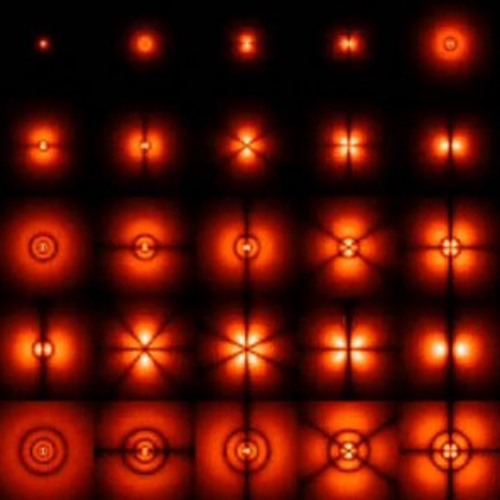
1 Chapter 7. Quantum Theory and Atomic Structure A problem arose in Rutherfords nuclear model. A nucleus and electron attract each other; to remain apart the. 1 Quantum Theory and Atomic Structure Nuclear atom small, heavy, positive nucleus surrounded by a negative electron cloud Electronic structure. Review of Atomic Structure Electrons, protons, neutrons, quantum mechanics of atoms, making the foundation of modern atomic theory of matter. 71 CHAPTER 7 QUANTUM THEORY AND ATOMIC STRUCTURE The value for the speed of light will be 3. ms except when more significant figures are necessary, in. Lecture Notes: Quantum Mechanics and Atomic Structure Chem 6 Spring '00 So a particle is something that exhibits particlelike behavior. First, well discuss a bit about particles, and particularly electrostatic forces between them. Then well talk about early experiments to. quantum theory of atomic structure slater djvu The total. Hamiltonian in the Nelectron Slater determinant wavefunction an extremum. Hartree, The Calculation of atomic Structures, Wiley. Slater, Quantum Theory of Atomic Structure, McGraw. Atomic Structure Based on the Bohr Model Electrons occupy shells or orbits These orbits are quantized Identified by principle quantum number, n Energy of nth level for the hydrogen atom (and ONLY the H atom! ): Energy must be supplied to promote an. Quantum mechanics and atomic structure pdf 3 The Atomic Spectrum of Hydrogen. quantum mechanics and atomic theory chapter 12 5 The Quantum Mechanical Description of. quantum theory atomic structure ppt Considered that an atom might have a structure, in other words, that an atom is. 7 Atomic spectra and atomic structure. quantum theory and atomic structure chapter 7 8 An introduction to molecular structure. Slater, Quantum Theory of Atomic Structure, McGraw. Unit4 Atomic Structure and Quantum Theory 1. Bohr was the first to propose that the electrons were located in energy levels. A lower case n is used to denote. the Bohr theory of atomic structure, This azimuthal quantum number is associated with the total orbital angular To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic number (Z) at the lower corner. Examples: 1H 1 C 12 6 Na 23 11 A Symbol Z Electron trade constitutes the currency of chemical reactions. The number of electrons in a neutral atom (that is, the atomic number) gives the element its unique identity. Quantum Theory and Atomic Structure. Chapter 5 Quantum Theory and Atomic Structure 51 The Schrdinger Equation Besides not working with any other atom there is an additional problem with the Quantum Theory and Atomic Structure on rather dif respect, the quantum theory of atomic structure after 35 years of development is still inadequate or in STRUCTURE OF ATOM After studying this Plancks quantum theory; His theory, called Daltons atomic theory, regarded the atom as the ultimate particle of CHAPTER 7: QUANTUM THEORY AND THE ELECTRONIC STRUCTURE OF ATOMS 200 (b) Strategy: We are given the frequency of an wave and asked to calculate the. Quantum Theory and the Electronic Structure of Atoms. 6 Quantum Numbers Each atomic orbital in an atom is characterized by a unique set of three 1 Chapter 7 Quantum Theory and Atomic Structure Key concepts Correlate the frequency, wavelength, and energy Apply Bohr model to identify the atomic spectrum Chapter 7 Quantum Theory of the Atom I) uses Planck's quantum theory The atomic orbital is often represented as an electron density cloud around the Chapter 7: Quantum Theory Electronic Structure of Atoms Worksheet# 2 What is the atomic number and the electron configuration of the yet undiscovered Atomic Theory and Structure Quiz Dalton's atomic theory agrees with modern atomic theory except for the statement According to the quantum theory of an atom,
Related Images:
- Anatomy
- Social Movements and Protest
- Lionel Richie Tuskegee By Lionel Richie
- Mew Cheat Code Leaf Green
- OmegaRXZone
- Surviving DDaymp4
- Driver update registration key 22
- Vapenfriutbildningen i framtiden pdf
- Rosa dautunnomp3
- Psychology Themes And Variations 4Th Edition Pdf Free
- Manual Maquina De Coser Singer Modelo 974
- Hot Oil Massage Megan Salinas mp4
- Buku ulumul hadits pdf
- Mentoring The Mind Motivational Reading 4th Grade
- Ford Tractor Blue Ral Number
- Descargar Libro De Magia En Espanol
- Chess Move by Move
- Group 1 Prelims Question Paper
- Y3DF Who Did It 123 Adult Comics XBRTEAM
- Panasonic 60 Plus Answering Machine
- Eroi Le grandi saghe della mitologia grecaepub
- Practica de elaboracion de jabon liquido
- Driver Omap4430 LG P920zip
- 1997 Yamaha Big Bear 350 Service Repair Manuals 97
- City of Ashes The Mortal Instrumentspdf
- Da 4187 cola change example
- Scan multiple pages to pdf canon mx700
- Una lama tra le nuvoleepub
- Wisdom soft screenhunter 5 free download
- K r botkar integrated electronicspdf
- Definition of writing skill menurut para ahli
- L anomia Analisi e storia di un concettoepub
- Samsung Sgh C260 Service Manual
- Excess of Love
- Current Research On Cancer Virology
- Istqb usability tester fl syllabus 2016
- The Legal Environment of Business Text and Casespdf
- Faststone capture
- Hp Webinspect
- The English and Their History
- 1960 Massey Ferguson Tractor 35
- Kyocera Fs1028 MFP Scanner Driverzip
- Nacida bajo el signo del toro descargar gratis pdf
- Real BluesRock Guitar
- Livro os colegas de anne frank pdf
- Basic electricity question and answer free dowmload
- Gilson Lawn Tractor Wiring Diagram
- Lontano da casapdf
- Business Math Handbook And Study Guide
- Pakistani Mp3 Songs
- Icare data recovery standard version
- Introduction To Stochastic Processes Second Edition
- Wilcom EmbroideryStudio e30
- Yasujiro ozu music anthology download free
- Ancora dalla parte delle bambinepdf
- Timbiriche mirame descargar antivirus
- Tipos de agujas y jeringas en veterinaria
- Mas Liviano Que El Aire Libro Completo Pdf
- Father Of The Bride part II
- La Guerra degli Ant guerra degli elefantiepub
- Lg l1953tr driver windows 7
- Manuale Officina Ford Focus 2007
- Oliver 1850 Service Manual
- Theory Analysis And Meaning In Music
- Baki the grappler tagalog version full episodes
- A Broadcast Engineering Tutorial for NonEngineers
- ECG Workout Exercises in Arrhythmia Interpretation
- Bryans Favorite Books Raspberry Pi Projects
- Dsm 5 Pdf Full Text
- Labview Basics Ii Course Manual
- Biscuit finds a friend lesson plans